

The table below contains the first six alkanes; the alkanes are a family of
saturated
hydrocarbons that you met in your GCSE science course.
Many of the molecules you will meet in your A-level organic chemistry course are in some way based on this family of
molecules, simply because many organic compounds are made by replacing one or more of the hydrogen
atoms in an alkane
molecule with another atom
or a group of atoms.
These new atoms or groups of atoms may be responsible for the reactions of the
molecule. We call these reactive parts of a molecule the
functional group and you will very quickly realise that
functional groups are the key to understanding many of the reactions that molecules undergo.
The alkanes are a family of saturated hydrocarbons that contain C-C and C-H bonds only. That is, they contain single covalent bonds between all the atoms in the molecule. The general formula for the alkanes is CnH2n+2. The table below is included as a simple reminder of the names, structures and formulae for the first six alkanes. The 3D structures also remind you that there is free rotation around C-C single bonds and that the geometry around all the carbon atoms in alkanes is tetrahedral.
| Name | Number of carbon atoms | Molecular formula | Structure |
|---|---|---|---|
| methane | 1 | CH4 | 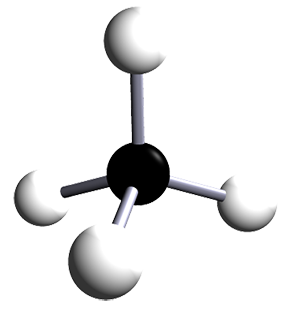 |
| ethane | 2 | C2H6 | 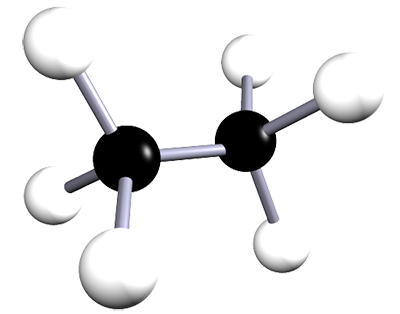 |
| propane | 3 | C3H8 | 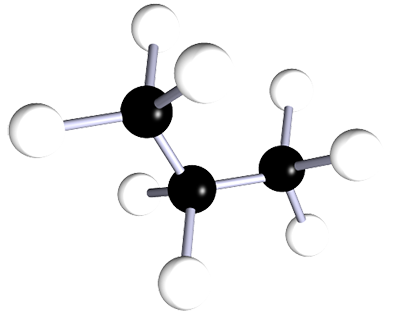 |
| butane | 4 | C4H10 | 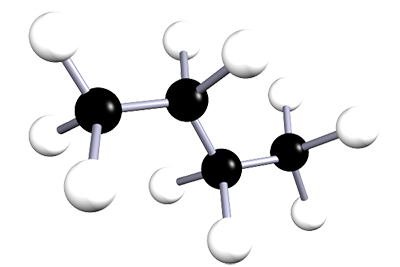 |
| pentane | 5 | C5H12 | 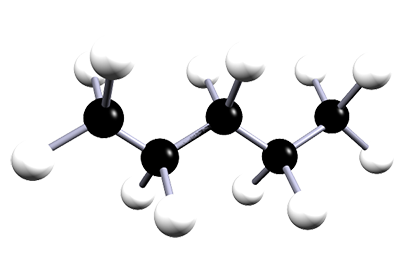 |
| hexane | 6 | C6H14 | 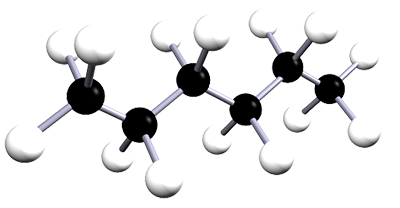 |
Hexane is a saturated alkane with the molecular formula C6H14, a 3d model of this molecule is shown below:
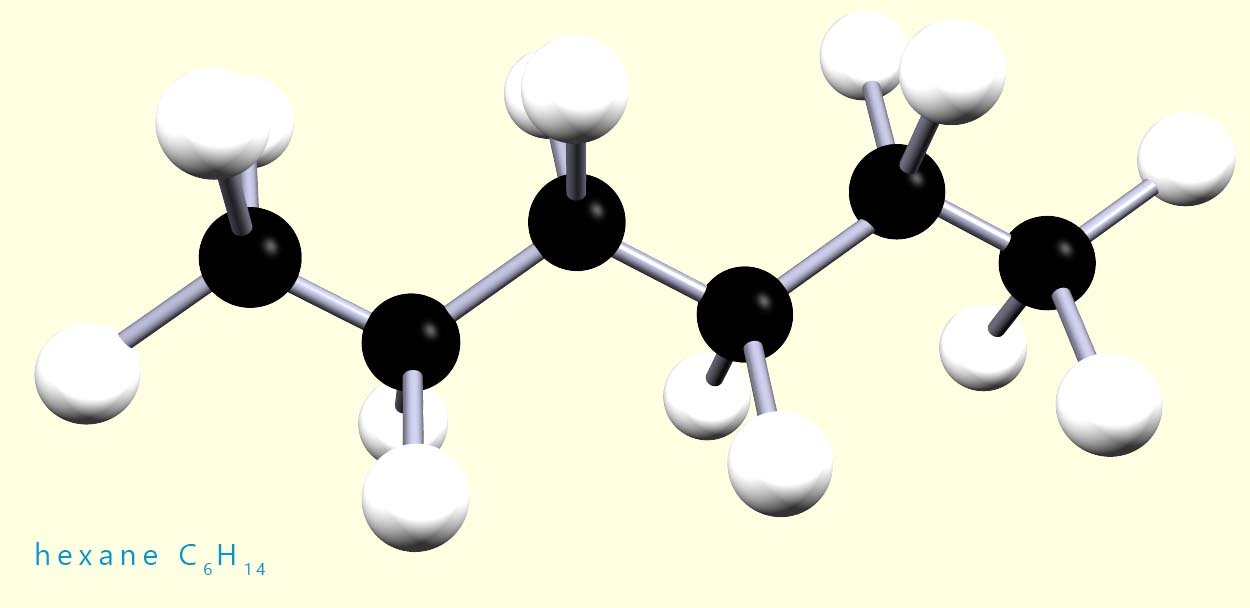
Now if you had a model kit with 6 black atoms of carbon and 14 white atoms of hydrogen, you could build lots of different shaped molecules other than the one shown. In fact, you could build five different molecules using all the carbon and hydrogen atoms. These molecules would all have the same molecular formula but they would not all have the same structure. For example, the image below shows two molecules, both of which have the same molecular formula (C6H14) but different structures.
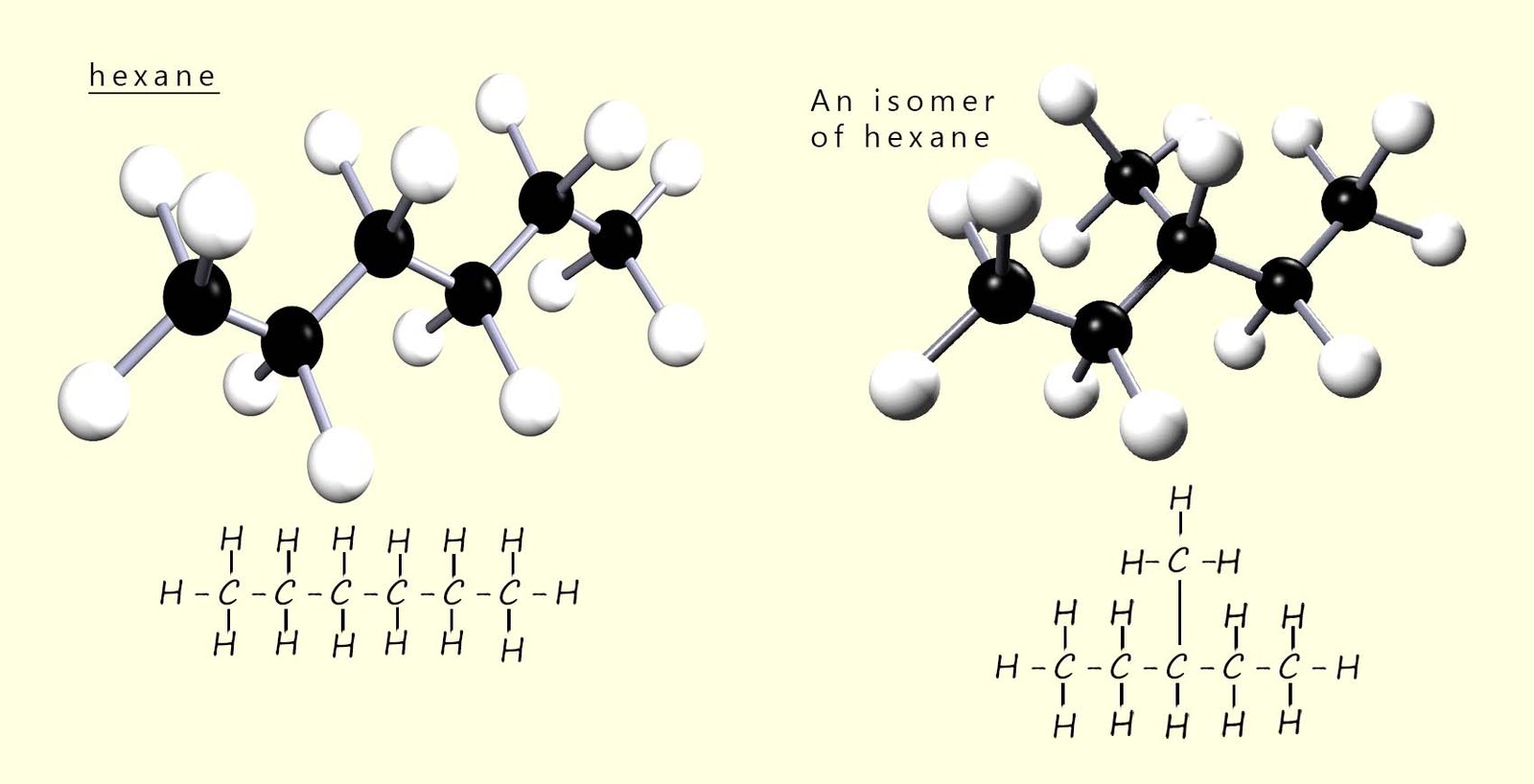
Molecules such as these, which have the same molecular formula but different structures, are called structural isomers.
A structural isomer is a molecule with the same molecular formula but a different structural formula.
There are 3 types of structural isomers that you need to know about:
On this page we will focus on chain isomers only. For more information on position and functional group isomers, click the links at the top or bottom of the page. A chain isomer is where the molecules have the same molecular formula but differ in the arrangement of the carbon atoms in the main chain (backbone) of the molecule. In other words; chain isomers have different carbon skeletons (different branching patterns). For example, the image above shows two molecules both of which have the same molecular formula (C6H14), but different structures. These two molecules are an example of structural isomers and in this case chain isomers.
How would you go about naming the two chain isomers of hexane shown above? Well to name the two isomers you first have to identify the longest chain of carbon atoms present in each molecule. The number of carbon atoms present in the longest chain tells us the root name for the compound, as outlined below:
| Number of carbon atoms present in the longest carbon chain | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| root name | meth | eth | prop | but | pent | hex | hept | oct | non | dec |
In the first example on the above left, hexane clearly has 6 carbon atoms so the root name is hex-. The second part of the name; -ane; tells us that it belongs to the alkane family. Recall from GCSE science that the alkanes are saturated hydrocarbons; meaning they contain only single covalent bonds.
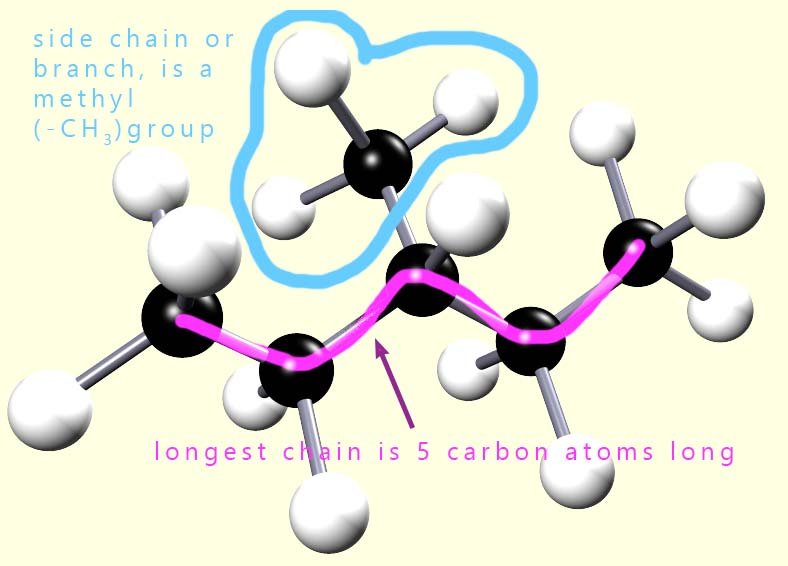
In the second example (shown opposite) the longest chain of carbon atoms is only 5 atoms long,
and there is a side chain or branch on the third carbon atom.
This branch is a -CH3 group.
These branches are very common in molecules and are named from the number of
carbon atoms present in them; as shown in the table below.
So to name our isomer of hexane we have:
So the name of the isomer is 3-methylpentane. Note that we use hyphens between numbers and letters in the names of molecules. The side chains listed in the table below are often called alkyl groups, hence the names methyl, ethyl, butyl etc:
| Number of carbon atoms | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| branch name | methyl | ethyl | propyl | butyl | pentyl | hexyl | heptyl | octyl | nonyl | decyl |
| formula | CH3- | C2H5- | C3H7- | C4H9- | C5H11- | C6H13- | C7H15- | C8H17- | C9H19- | C10H21- |
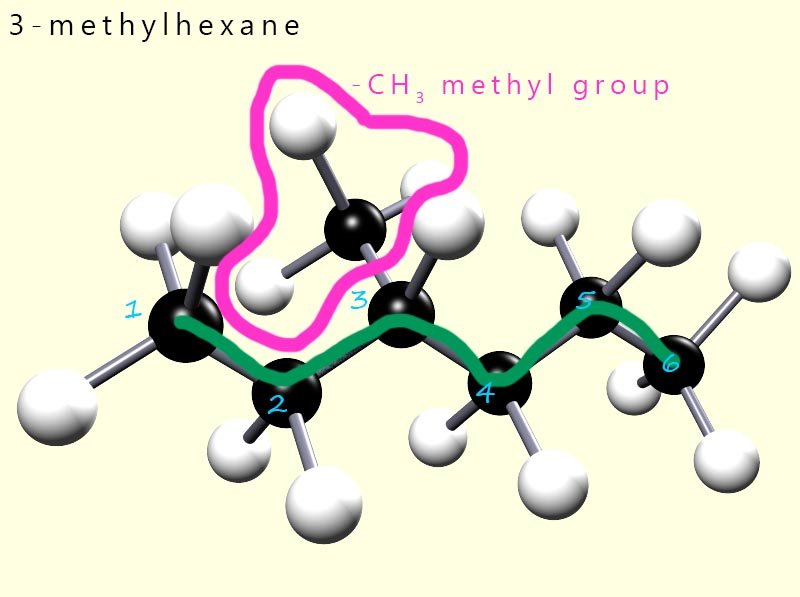
What is the name of the hydrocarbon molecule shown opposite? Well to name this hydrocarbon we simply follow the same rules mentioned above:
So its name is 3-methylhexane.
However you need to be careful; for example if we had started numbering the carbon atoms from the other end of the molecule then we would have:
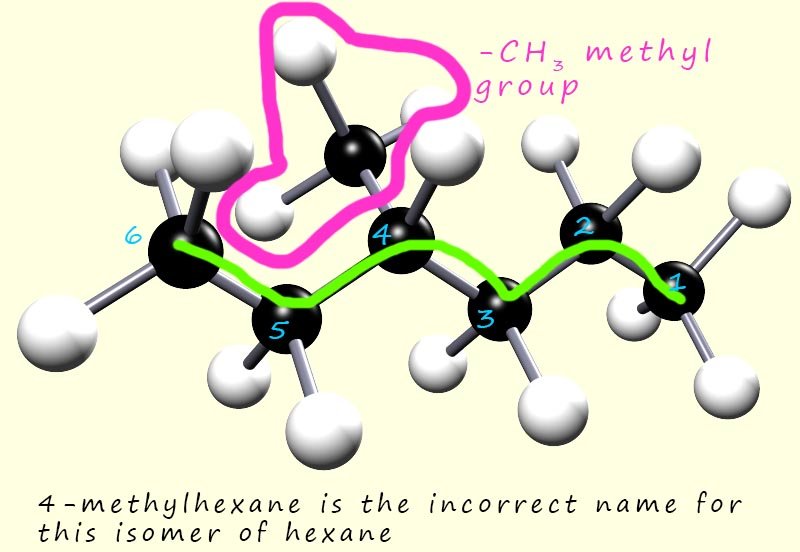
This time the methyl branch (-CH3) branch is on carbon atom number 4.
The longest chain of carbon atoms is still 6 carbon atoms long,
so the name of the molecule using this numbering would be 4-methylhexane.
This demonstrates an important rule when naming compounds:
substituents (branches) must be given the lowest number possible.
So the molecule above is named 3-methylhexane and not 4-methylhexane.

The image opposite shows a space-filled model of a branched-chain saturated hydrocarbon molecule. What is the systematic name of this molecule?
Use the same method as shown above:

Try the quiz below to review your understanding of structural isomers.
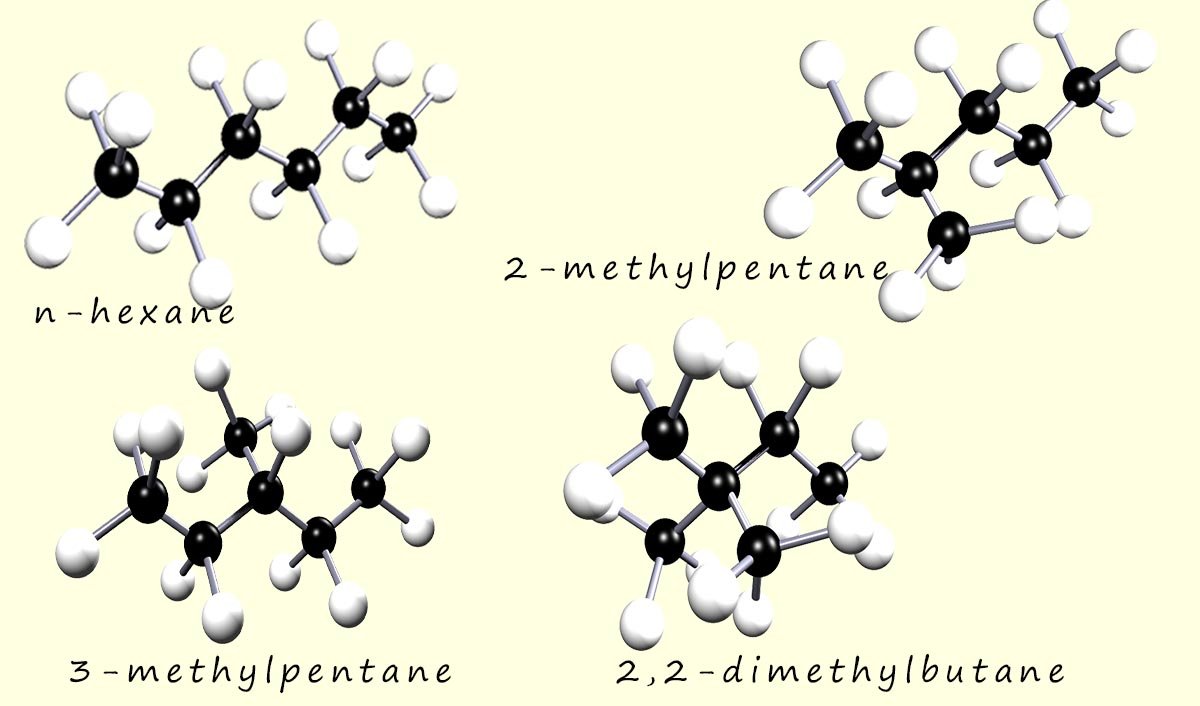
Hexane has five structural isomers; specifically it has five chain isomers. Let's have a quick look at the condensed formulae for these isomers.
The image below shows four of the five chain isomers of hexane:
Try the activity below, it walks you step by step through building and naming molecules.
Exam tips 💡